Reporting a problem with a medicine
Key facts
- Every medicine, including 'natural' medicines, can possibly cause a problem for some people.
- Problems with medicine and therapeutic products may include adverse events (side effects) and problems with manufacturing, packaging or distribution.
- The sale of counterfeit medicines is another type of problem, and should be reported.
- You can report adverse events to the Therapeutic Goods Administration (TGA), or you can ask your doctor or pharmacist to do it for you.
- Reporting problems with your medicine helps monitor its safety and contributes to public health.
Why should I report a problem with a medicine?
In Australia, the Therapeutic Goods Administration (TGA) is responsible for monitoring the safety of medicines.
When a medicine is first made available, information about its safety and function comes from clinical trials. While these clinical trials report information about possible problems and side effects, not all the possible problems can be identified.
By reporting a problem that you have with your medicine, you help monitor its safety.
The types of medicines and equipment you can report on to the TGA include:
- medicines prescribed by a doctor, including vaccines
- over-the-counter' medicines bought at a pharmacy, supermarket or online
- medicines that are complementary or 'natural', including vitamins and minerals
- medical devices
What are potential problems I could have with a medicine?
Potential problems with medicines may include adverse events, packaging or handling issues, as well as counterfeit products.
Adverse events, including side effects
A medicine or vaccine can sometimes cause unintended or unwanted effects, called 'adverse events'. These may include signs, symptoms or a disease that happens during or after using the medicine or vaccine.
Adverse events include unintended side effects, called adverse drug reactions or adverse effects. Common and known side effects are listed in the Consumer Medicines Information (CMI) for each medicine. Some side effects are more likely than others. You may not experience any side effects at all.
Adverse events can also involve problems or accidents with medical devices used to deliver a medicine. For example, an insulin injection device. Problems may include:
- accidents that have happened
- things that could happen
- near misses (problems that could have caused serious harm or death without intervention)
Not all adverse events happen because of a problem with the medicine or medical device itself. They can also be caused by:
- an allergy you may have to the medicine
- incorrect use, such as taking the wrong dose
- the way the dose of a medicine is started, then changed
It is possible for your medicine to interact with another medicine you may be taking. This can affect the way they work or how you react to them. Certain foods or drinks can also interact with medicines and lead to an adverse event.
Packaging, handling or storage of medicine
Possible problems include any defects that may happen during the manufacture or distribution of a medicine. Issues may involve an entire batch or a single package of the medicine. These defects can affect their:
- safety
- quality
- how well the medicine works
Defects may include:
- incorrect labels
- changes in the medicine's colour or appearance
- faulty or tampered packaging
- contamination
Packaging problems may lead to confusion when purchasing a medicine. Generic-looking packaging or medicines with similar-sounding names can lead to medicine mix-ups and errors when selecting your medicine.
Counterfeit (fake) medicines and questionable practices
Counterfeit medicines and medical devices are products that may look like the real product but may:
- be sold without approval in Australia
- be illegally copied and produced by another manufacturer
- contain incorrect amounts of active ingredients or none at all
- be toxic or unsafe
Counterfeit products can be harmful and may not work as intended. Issues may also arise from misleading advertising or claims made by a manufacturer. Learn how to spot a fake medicine.
What should I do if I have a problem with a medicine?
If you experience side effects, notice a problem or have concerns about your medicine or medical device:
- If it's an emergency, call triple zero (000) for an ambulance.
- Seek advice from a health professional such as your doctor or pharmacist.
- For suspected overdosing or poisoning, call the Poisons Information Centre on 13 11 26.
- You can also report a problem directly to the TGA, which regulates medicines in Australia.
For non-emergency situations, you can:
- Inform your doctor or health professional.
- Call the Adverse Medicines Events (AME) Line on 1300 633 424
- Report the problem through the TGA website
FIND A HEALTH SERVICE — The Service Finder can help you find doctors, pharmacies, hospitals and other health services.
Can I report a problem with a medicine to my healthcare provider?
If you are having any problems with a medicine, it's a good idea to tell your doctor or pharmacist about it. They can help if you're experiencing an adverse event. It may be needed to:
- stop the medicine
- change the dose
- switch to another medicine
Your doctor or pharmacist can report the problem to the TGA for you. Your personal information will stay confidential and your privacy will be respected.
They will also update your personal health records with information about any reactions or side effects you've had.
Can I report a problem with a medicine to the TGA?
You can report a problem with a medicine directly to the TGA. They monitor the safety of medicines, including adverse events. The TGA can then ensure this information is available to healthcare professionals and people who take the medicine.
Reports can be made by anyone, including:
- pharmaceutical companies who make and sell the medicine
- doctors, pharmacists and other healthcare professionals
- hospitals and health departments
- people who take the medicine
You do not need to be certain that you have a problem or are experiencing an adverse event. Suspected adverse events are also encouraged to be reported.
The TGA investigates these reports and publishes the findings in the Database of Adverse Event Notifications (DAEN).
How do I report a problem to the TGA?
- You can report a problem with a medicine or vaccine directly to the TGA.
- An adverse event with a medicine can be reported on the TGA website or call 1300 MEDICINE (1300 633 424). A pharmacist can report your problem for you.
- A problem with a medical device can be reported on the TGA website.
- Manufacturing, storage or handling defects can be reported on the TGA website.
- Unapproved or questionable medicines can be reported on the TGA website.
What information do I need to report?
When you report a problem, it's a good idea to keep any packaging, prescriptions or documents that came with the medicine. Make note of details, such as:
- product name and AUST number
- manufacturer details
- catalogue, lot, batch or serial numbers
- expiry dates
It is also important to keep notes about your experience with the medicine. Consider taking photos of the product and your symptoms. Important details to include are:
- when and where you bought the product
- how you stored it
- when you first noticed the problem or side effect
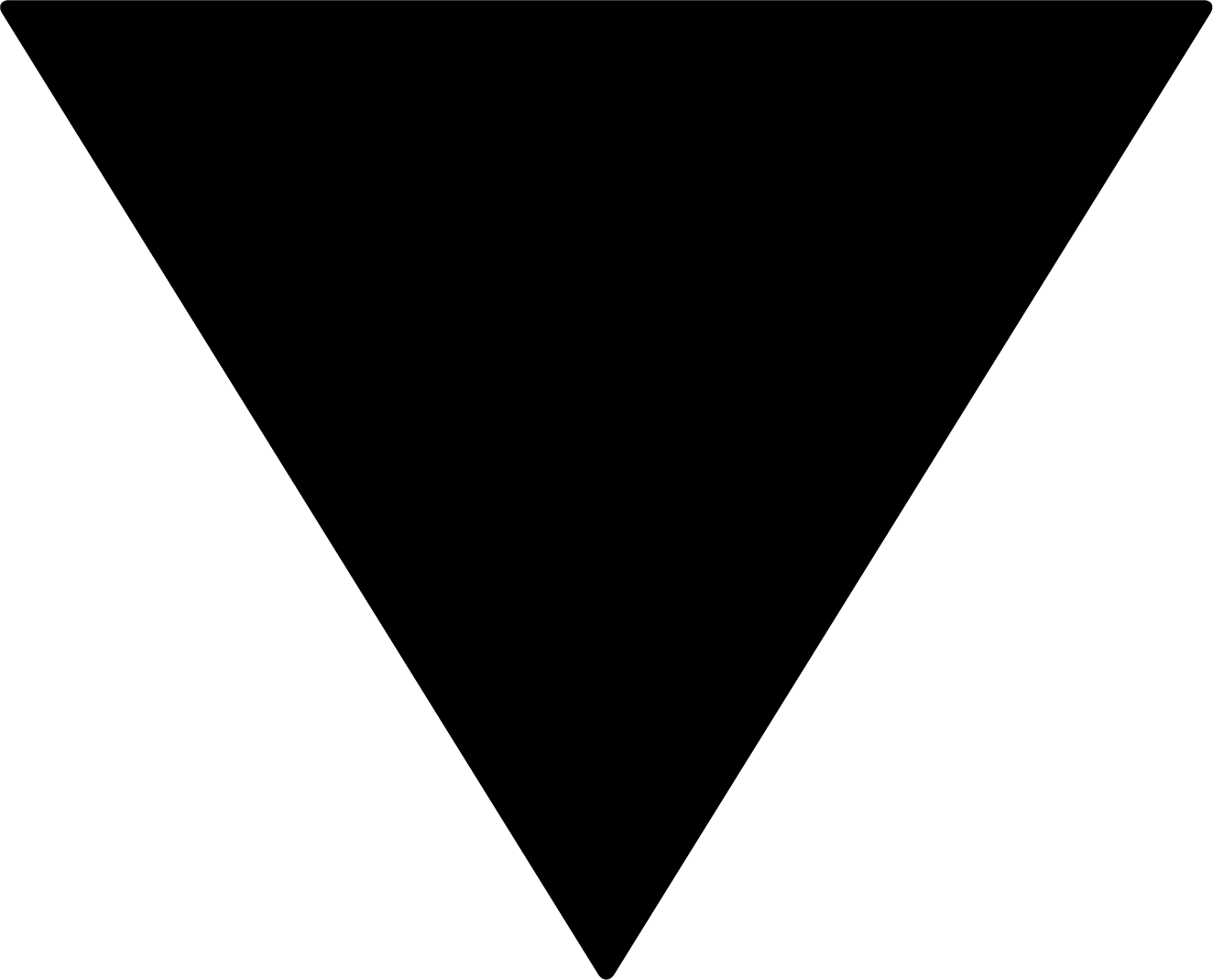
What is the Black Triangle Scheme?
The TGA has introduced the Black Triangle Scheme. A black triangle symbol appears on documents related to a newly released medicine or a medicine being used in a new way. This serves a reminder that you should monitor and report any problems that may happen when you take the medicine.
The black triangle does not mean there are concerns or known safety problems with the medicine. Sometimes, an adverse event will not happen during the product's clinical trials. They may only be noticed and monitored as the medicine is used by more people once it has been released.
You will see this black triangle on medicine information documents for newer medicines.
Resources and support
Find out more about reporting a problem with a medicine from:
- your doctor
- your pharmacist
- the TGA website
- the medicines.org.au website
If you have a question about your medicine, call the Medicines Line on 1300 MEDICINE (1300 633 424) and speak to a pharmacist.
The TGA uses an upside-down black triangle to encourage reporting of any problems with new medicines. If a medicine has a black triangle, you will find it in the medicine's CMI.
You can also call the healthdirect helpline on 1800 022 222 (known as NURSE-ON-CALL in Victoria). A registered nurse is available 24 hours a day, 7 days a week.
Do you prefer to read in languages other than English?
If you speak a language other than English, you can use the Translating and Interpreting Service (TIS National) to report problems with a medicine.
Learn more here about the development and quality assurance of healthdirect content.
Last reviewed: November 2024

















