Lipigem TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: gemfibrozil
Pack: Lipigem 600 mg tablet, 60, bottle
Brand name
(ARTG)
: LIPIGEM gemfibrozil 600mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
LIPIGEM is indicated as an adjunct to diet and other therapeutic measures for: *Severe hypertriglyceridaemia (Type IV and V) who present a risk of pancreatitis and who do not respond adequately to a determined dietary effort to control them. *Dyslipidaemia associated with diabetes. *Reduction of risk of coronary heart disease in patients with Type IIa and IIb hypercholesterolaemia. Because of potential toxicity such as malignancy, gallbladder disease, abdominal pain leading to appendectomy and other abdominal surgeries, an increased incidence in noncoronary mortality, and the 29% increase in all-cause mortality seen with the chemically and pharmacologically related drug, clofibrate, the potential benefits of gemfibrozil in treating Type IIa patients with elevations of LDL-cholesterol only are not likely to outweigh the risks. In a subgroup analysis of patients in the Helsinki Heart Study with above-median HDL-cholesterol values at baseline (greater than 1.2mmol/L), the incidence of serious coronary events was similar for gemfibrozil and placebo subgroups. NOTE: LIPIGEM IS INDICATED WHEN EXERCISE, WEIGHT LOSS AND SPECIFIC DIETARY OR OTHER NONDRUG MEASURES SUCH AS LIMITING ALCOHOL INTAKE HAVE FAILED. OTHER MEDICAL DISORDERS SUCH AS HYPOTHYROIDISM AND DIABETES SHOULD BE CONTROLLED AS MUCH AS POSSIBLE. PERIODIC DETERMINATION OF SERUM LIPIDS SHOULD BE OBTAINED DURING TREATMENT WITH LIPIGEM. THE DRUG SHOULD BE WITHDRAWN OR ADDITIONAL THERAPY INSTITUTED IF THE LIPID RESPONSE IS DEEMED INADEQUATE AFTER THREE MONTHS.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
- 19 mm x 9.2 mm deep convex, white, film coated oval shape tablet, debossed,GL600 on one side and Greek alpha symbol on the other.
Images
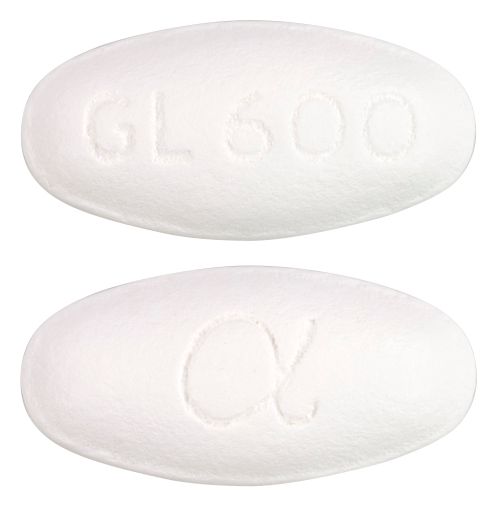

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Shelf lifetime is 18 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 60 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient gemfibrozil
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









