Afinitor TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: everolimus
Brand name
(ARTG)
: AFINITOR everolimus 2 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
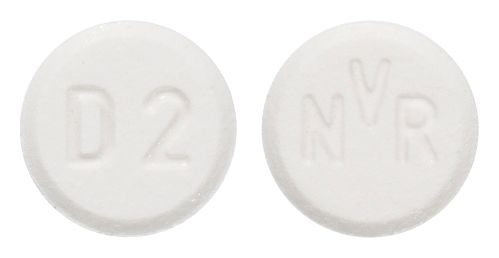
- White to slightly yellow round flat tablet with bevelled edge engraved D2 on one side and NVR on the other
Images

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 2 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D2 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 2 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D2 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 2 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D2 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 2 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D2 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 3 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
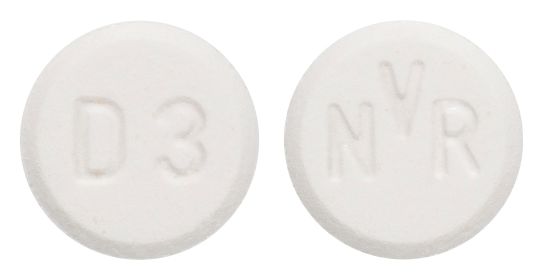
- White to slightly yellow round flat tablet with bevelled edge engraved D3 on one side and NVR on the other
Images

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 3 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D3 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 3 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D3 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 3 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D3 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 3 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D3 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 5 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
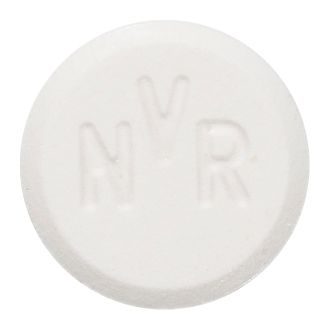
- White to slightly yellow round flat tablet with bevelled edge engraved D5 on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 5 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D5 on one side and NVR on the other
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 5 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D5 on one side and NVR on the other
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 5 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D5 on one side and NVR on the other
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 5 mg dispersible tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, dispersible
- Oral
- White to slightly yellow round flat tablet with bevelled edge engraved D5 on one side and NVR on the other
Images



Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 pack
- 120 pack
- 30 pack
- 50 pack
- 60 pack
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 5 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
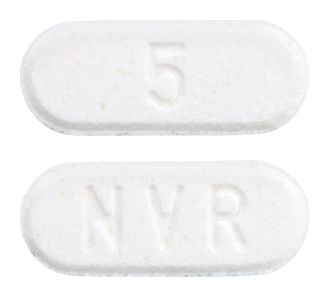
- White to slightly yellowish, elongated tablet with a bevelled edge. One side debossed with 'NVR' and the other side with '5'.
Images

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 Tablets pack
- 120 Tablets pack
- 30 tablets pack
- 50 Tablets pack
- 60 Tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 10 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White to slightly yellowish, elongated tablet with a bevelled edge. One side debossed with 'NVR' and the other side with 'UHE'.
Images
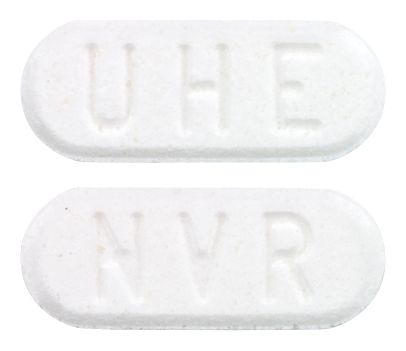

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 100 Tablets pack
- 120 Tablets pack
- 30 tablets pack
- 50 Tablets pack
- 60 Tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: AFINITOR everolimus 2.5 mg tablet blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Afinitor is indicated for the treatment of:, Postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. Progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. Progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. Advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. Subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. Patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery.Afinitor is indicated for the: ? Treatment of postmenopausal women with hormone receptor-positive, HER2 negative advanced breast cancer in combination with exemestane after failure of treatment with letrozole or anastrozole. ? Treatment of progressive, unresectable or metastatic, well or moderately differentiated neuroendocrine tumours (NETs) of pancreatic origin. ? Treatment of progressive, unresectable or metastatic, well-differentiated, non-functional neuroendocrine tumours (NET) of gastrointestinal or lung origin in adults. ? Treatment of advanced renal cell carcinoma after failure of treatment with sorafenib or sunitinib. ? Treatment of subependymal giant cell astrocytoma (SEGA) associated with tuberous sclerosis complex (TSC) who require therapeutic intervention but are not candidates for curative surgical resection. ? Treatment of patients with tuberous sclerosis complex (TSC) who have renal angiomyolipoma not requiring immediate surgery. ? Adjunctive treatment of patients aged 2 years and older with TSC and associated refractory seizures.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet
- Oral
- White to slightly yellowish elongated tablet with a bevelled edge, engraved with LCL on one side and NVR on the other
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is Schedule 4 : Prescription Only Medicine.
- 10 pack
- 30 pack
- 90 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient everolimus
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









