Lanvis TM
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: tioguanine
Pack: Lanvis 40 mg tablet, 25, bottle
Brand name
(ARTG)
: LANVIS tioguanine 40mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
INDICATIONS AS AT 10 OCTOBER 2005: Acute myeloblastic leukaemia. Less commonly, chronic granulocytic (myelocytic, myeloid, myelogenous) leukaemia. Although superior results are generally obtained with busulphan (Myleran) in the treatment of chronic granulocytic leukaemia. LANVIS may be useful during blast crises or periods of thrombocytopenia induced by busulphan or other therapy. A degree of cross resistance exists between LANVIS (thioguanine) and Puri-Nethol (mercaptopurine) and generally it is not to be expected that patients who no longer respond to mercaptopurine will respond to thioguanine or vice versa. Unlike mercaptopurine the detoxification of LANVIS is not dependent on xanthine oxidase, hence therapy with LANVIS is not affected by the xanthine oxidase inhibitor, allopurinol (Zyloprim). Recent evidence suggests that LANVIS is particularly useful in concurrent or sequelist combination with other antineoplastic drugs, e.g., cytosine arabinoside. Not effective for the treatment of chronic lymphocytic leukaemia or solid tumours. The aim of therapy is to achieve normal appearance of the bone marrow and pheripheral blood. It is recognised, however, that in treating acute leukaemia, one may not always be able to achieve complete remissions and must be satisfied with partial improvement.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White to off-white tablet, round, biconvex scored and marked "T40" on one side and plain on the other
Images
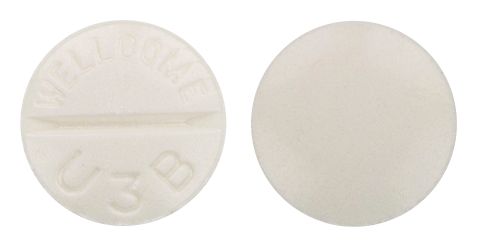

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in a Dry Place
- Shelf lifetime is 5 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 25 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient tioguanine
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









