Eskazole TM
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: albendazole
Brand name
(ARTG)
: ESKAZOLE albendazole 400mg chewable tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
HYDATID DISEASE: Albendazole is indicated for the treatment of hydatid cysts caused by E. granulosus in adults and children over 6 years of age, where surgical intervention is not feasible because of anatomic site or the presence of multiple cysts. Albendazole shows greatest efficacy in the treatment of liver, lung and peritoneal cysts. Experience with bone cysts or those in the heart or central nervous system is limited, but cases of successful treatment with prolonged course of albendazole have been reported. Albendazole may also be used as an adjunct to surgical excision of hydatid cysts either: 1) prior to surgical intervention, or 2) post-operatively, if pre-operative treatment was too short (less than two separate 28-day cycles) or if viable cysts are found at surgery. LARVAL TAENIASIS (NEUROCYSTICERCOSIS): Albendazole is effective in the treatment of neurocysticercosis (NCC) in courses as short as 7 days. OTHER INDICATIONS: There is also evidence that albendazole is effective against Capillaria philippinensis in courses of 10 days.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, chewable
- Oral
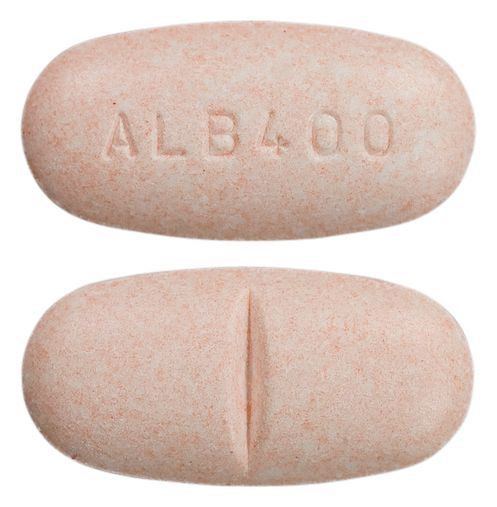
- Mottled pale orange rounded oblong biconvex tablets with a score line on one side and embossed "ALB400" on the reverse, with a characteristic fruity odour.
Images

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Shelf lifetime is 5 Years.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 60 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient albendazole
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









