Dilaudid TM
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: hydromorphone
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 2mg/1mL injection ampouleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID injection is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Injection, solution
- Intramuscular
- Clear, colourless to pale yellow solution.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Light
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 5 x 1mL ampoules pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 2mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Light orange, round, flat faced tablets with bevelled edges, with the number "2" on one side and a "D" on the other side.
Images
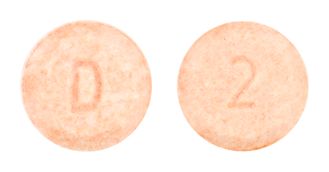

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 2mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Light orange, round, flat faced tablets with bevelled edges, with the number "2" on one side and a "D" on the other side.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 2mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Light orange, round, flat faced tablets with bevelled edges, with the number "2" on one side and a "D" on the other side.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 2mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Light orange, round, flat faced tablets with bevelled edges, with the number "2" on one side and a "D" on the other side.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 4mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- light yellow, round, flat-faced tablets, with bevelled edges, with the number "4" on one side and a "D" on the other side;
Images
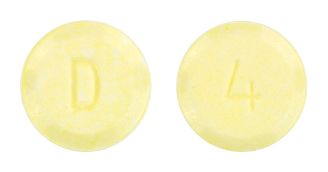

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 4mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- light yellow, round, flat-faced tablets, with bevelled edges, with the number "4" on one side and a "D" on the other side;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 4mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- light yellow, round, flat-faced tablets, with bevelled edges, with the number "4" on one side and a "D" on the other side;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 4mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- light yellow, round, flat-faced tablets, with bevelled edges, with the number "4" on one side and a "D" on the other side;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 8mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White triangular shaped tablet, bisected on one face, with a "D" on each side of the bisecting line, and with the number "8" on the other face;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 8mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White triangular shaped tablet, bisected on one face, with a "D" on each side of the bisecting line, and with the number "8" on the other face;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 60 tablets pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 8mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White triangular shaped tablet, bisected on one face, with a "D" on each side of the bisecting line, and with the number "8" on the other face;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 8mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- White triangular shaped tablet, bisected on one face, with a "D" on each side of the bisecting line, and with the number "8" on the other face;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 4mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- light yellow, round, flat-faced tablets, with bevelled edges, with the number "4" on one side and a "D" on the other side;
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems
Brand name
(ARTG)
: DILAUDID hydromorphone hydrochloride 2mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DILAUDID is indicated for the short-term management of severe pain for which other treatment options have failed, are contraindicated, not tolerated or are otherwise inappropriate to provide sufficient management of pain.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, uncoated
- Oral
- Light orange, round, flat faced tablets with bevelled edges, with the number "2" on one side and a "D" on the other side.
Images


Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Shelf lifetime is 3 Years.
Do I need a prescription ?
(ARTG)
These medicines require authorisation for prescription from your doctor. It is Schedule 8 : Controlled Drug.
- 10 pack
- 100 tablets pack
- 20 tablets pack
- 500 tablets pack
- 60 tablets pack
Over 65 ?
(AHT)
This medicine contains the active ingredients:
If you are over 65 years of age, there may be specific risks and recommendations for use of this medicine. Please discuss your individual circumstances with your pharmacist, doctor or health professional. For more information read our page on medication safety for older people.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient hydromorphone
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









