Dovato 50/300 TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: dolutegravir + lamivudine
Pack: Dovato 50/300 tablet, 30, bottle
Brand name
(ARTG)
: DOVATO 50/300 dolutegravir (as sodium) 50 mg/lamivudine 300 mg tablet bottleConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
DOVATO (a fixed dose combination of dolutegravir and lamivudine) is indicated for the treatment of Human Immunodeficiency Virus-1 (HIV-1) infection in adults and adolescents (from 12 years of age weighing at least 40kg): in antiretroviral treatment-naïve patients with no antiretroviral treatment history who have no known or suspected resistance to either antiretroviral component; or to replace the current antiretroviral regimen in those who are virologically suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment failure and with no known or suspected resistance to the integrase inhibitor class or lamivudine (see section 5.1 PHARMACODYNAMIC PROPERTIES, Clinical trials).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Tablet, film coated
- Oral
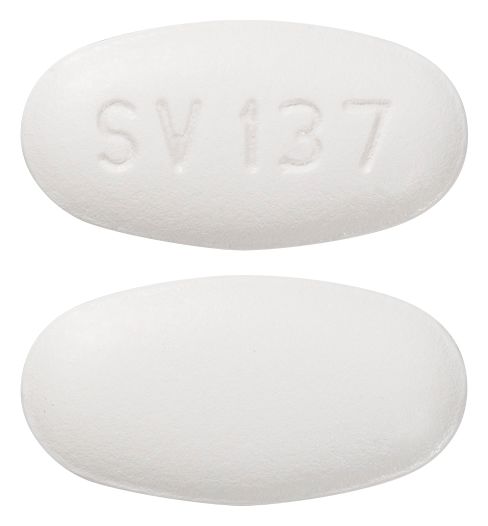
- Oval, biconvex, white, film-coated tablet, debossed with SV 137 on one face
Images

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Store in Original Container
- Shelf lifetime is 24 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 30 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient dolutegravir + lamivudine
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









