Rydapt TM
Listen to the Pronunciation:
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: midostaurin
Brand name
(ARTG)
: RYDAPT midostaurin 25 mg soft capsule blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
Listen to the audio transcription of the CMI leaflet.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Rydapt is indicated:, in combination with standard anthracycline and cytarabine induction and cytarabine consolidation chemotherapy, followed in patients in complete response by single agent maintenance therapy for adult patients with newly diagnosed acute myeloid leukemia (AML) who are FLT3 mutation-positive for the treatment of adult patients with aggressive systemic mastocytosis (ASM), systemic mastocytosis with associated haematological neoplasms (SM-AHN), or mast cell leukaemia (MCL).
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, soft
- Oral
- Pale orange, oblong capsule with red imprint PKC NVR
Images
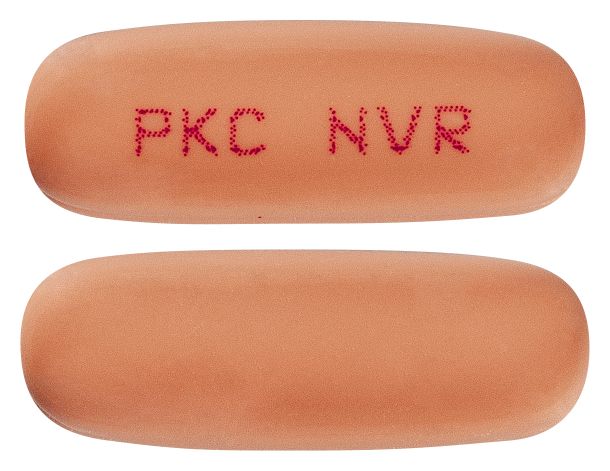

Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 30 degrees Celsius
- Protect from Moisture
- Shelf lifetime is 36 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 112 pack
- 56 pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient midostaurin
You should seek advice from your doctor or pharmacist about taking this medicine. They can help you balance the risks and the benefits of this medicine during pregnancy.
Reporting side effects
This medicine is under additional monitoring as it is new or being used in a different way. You can help identify new safety information by reporting any side effects you may get.
- You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems.
- For more information on the Black Triangle Scheme and how to report side effects, see www.tga.gov.au/black-triangle-scheme









