Calindamin TM
You should seek medical advice in relation to medicines and use only as directed by a healthcare professional. Always read the label. If symptoms persist see your healthcare professional.
Active ingredients: clindamycin
Brand name
(ARTG)
: CALINDAMIN Clindamycin (as hydrochloride) 150 mg capsules blister packConsumer Medicine Information (CMI)
Read the CMI leaflet for facts you need to know before, during and after taking your medicine.
For more information about CMIs and how to read them, please visit How to read Consumer Medicine Information (CMI).
What this medicine is used for
(ARTG)
Clindamycin hydrochloride capsules are indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. Clindamycin capsules are also indicated in the treatment of serious infections due to susceptible strains of streptococci, pneumococci and staphylococci. Its use should be reserved for penicillin-allergic patients or other patients for whom, in the judgement of the physician, a penicillin is inappropriate. Anaerobes Serious respiratory tract infections such as empyema, anaerobic pneumonitis and lung abscess; serious skin and skin structure infections; septicaemia; intra-abdominal infections such as peritonitis and intra-abdominal abscess (typically resulting from anaerobic organisms resident in the normal gastrointestinal tract); infections of the female pelvis and genital tract such as endometritis, non-gonococcal tubo-ovarian abscess, pelvic cellulitis and post-surgical vaginal cuff infection. Streptococci Serious respiratory tract infections; serious skin and skin structure infections, septicaemia. Staphylococci Serious respiratory tract infections; serious skin and skin structure infections; septicaemia; acute haematogenous osteomyelitis. Pneumococci Serious respiratory tract infections. Adjunctive Therapy In the surgical treatment of chronic bone and joint infections due to susceptible organisms. Indicated surgical procedures should be performed in conjunction with antibiotic therapy. Bacteriological studies should be performed to determine the causative organisms and their susceptibility to clindamycin.
How to use this medicine
(ARTG)
This medicine contains one component only.
Component :
- Capsule, hard
- Oral
- A hard gelatin capsule, size 1 white/white containing white crystalline powder, with marking "CLIN 150"
Images
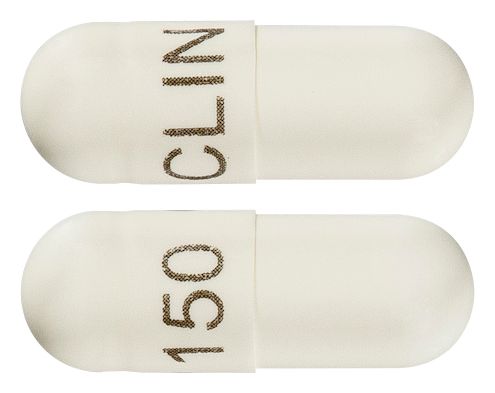
Images © Medicines Information Pty Ltd.
Storage conditions
(ARTG)
- Store below 25 degrees Celsius
- Protect from Light
- Store in Original Container
- Shelf lifetime is 48 Months.
Do I need a prescription ?
(ARTG)
These medicine packs are available from a pharmacist and requires a prescription. It is
- 100 capsules pack
- 24 capsules pack
Is this medicine subsidised ?
(PBS)
This medicine was verified as being available on the PBS (Pharmaceutical Benefits Scheme) on January, 6 2026. To learn more about this subsidy, visit the Pharmaceutical Benefits Scheme (PBS) website.
Pregnant or planning a pregnancy ?
(AHT)
For the active ingredient clindamycin
This medicine is generally considered safe during pregnancy if taken as directed. During pregnancy, you should discuss your medicine use with your doctor or pharmacist.
Reporting side effects
You can help ensure medicines are safe by reporting the side effects you experience.
You can report side effects to your doctor, or directly at www.tga.gov.au/reporting-problems









